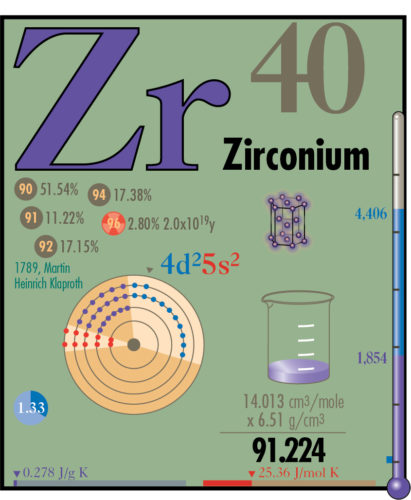
Below are some of the properties being presented for each element.
Electron configuration
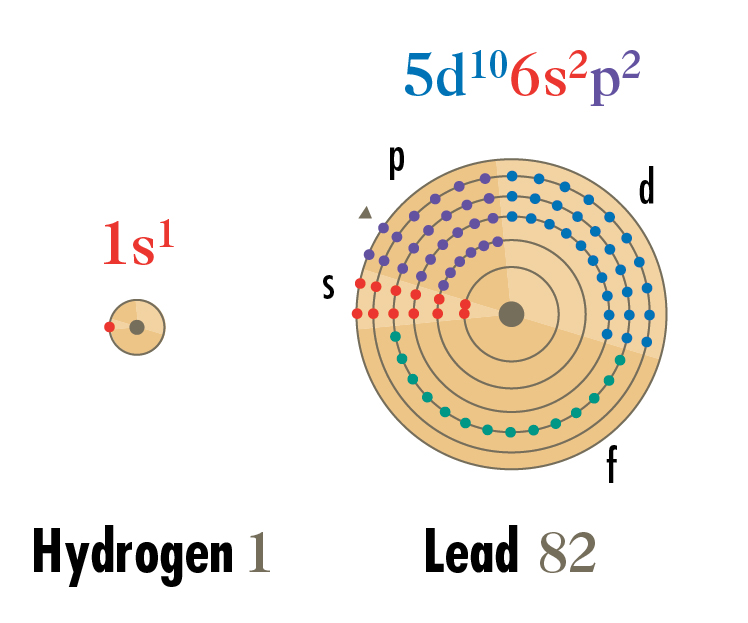
Electrons are diagrammed placing electrons according to the shells and energy levels, represented in pie shapes. Electrons in levels s, p, d and f are also color coded.
Atomic volume, density and weight
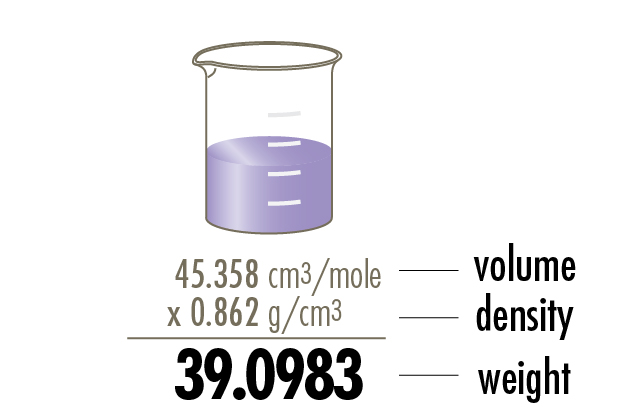
Atomic volume is demonstrated using a 100 ml beaker. The density and weight values are shown in mathematical multiplication format to portray their relationship.
Potassium, K 19, is shown.
Melting and boiling points
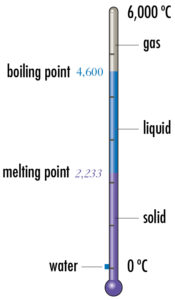
Melting and boiling points of the elements are shown using a thermometer. Its scale ranges from absolute zero to 6,000 ºC. The values are shown, with solid, liquid and gas states being color coded. For reference a small blue square shows the range of water, 0 ºC to 100 ºC.
Hafnium, Hf 72, is shown.
Electronegativity
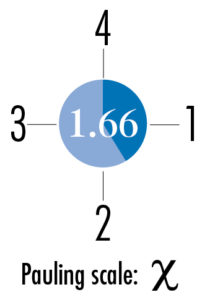
Electronegativity indicates how strongly an atom attracts the bonding electrons of another atom. The Pauling scale is used to indicate the strength, which ranges approximately between 0.7 and 4.0. Electronegativity is typically denoted by the Greek letter Chi.
The Pauling number is shown here overlaid on a pie chart that illustrates its strength. In general elements toward the lower left corner of the table have lower levels of electronegativity, elements toward the upper right corner have higher levels. Fluorine has the highest level at 3.98.
Chromium, Cr 24, is shown.
Crystal structures
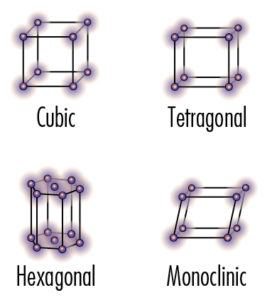
Crystal structures are show for each element. There are seventeen in total, four are shown here.
Specific heat and molar heat capacities
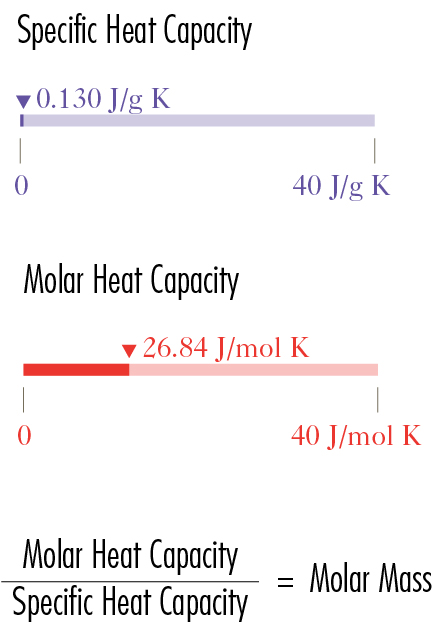
Specific heat and molar heat capacities are indicated along a bar chart at the bottom of each cell.
The SI unit for specific heat is: J/g K (joules per gram Kelvin).
The SI unit for molar heat is: J/mol K (joules per mole Kelvin)
Example of a cell